Prescription Drug Injuries and Deaths Higher
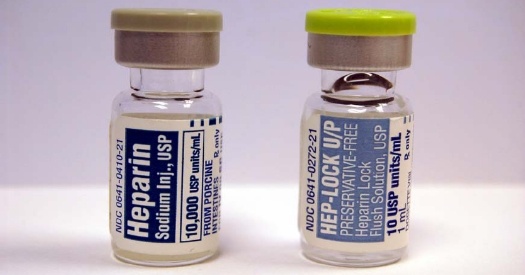
A health industry watchdog group said the number of serious drug reactions and deaths reported to the government shot up in the first three months of this year to set a new record. The Food and Drug Administration received nearly 21,000 reports of serious drug reactions, with 4,825 deaths and 21,000 injuries which occurred in the first three months of 2008 with drugs heparin and varenicline cited as the most dangerous said an analysis of federal data by the Institute for Safe Medication Practices.
The drug heparin is a blood thinner associated with 779 injuries and 238 deaths while varenicline sold under the brand name Chantix is a new anti smoking drug from Pfizer linked to 3325 serious injuries and 112 deaths. The heparin cases were associated with contaminated lots of the drug imported from China and the number reduced once the problem was recognized and addressed.
Pfizer released a statement to say, "Based on the [the] totality of data, we stand by the efficacy and safety profile of Chantix when used as directed," the statement said. "Chantix labeling accurately reflects its efficacy and safety event reports and clinical trial data. There are few things that provide greater health benefits than quitting smoking. Pfizer is committed to reducing the prevalence of smoking globally. As part of that mission, we want to increase peoples' understanding of the dangers of smoking and the benefits of quitting."
Earlier this year, the FDA warned that Chantix may be linked to psychiatric problems, including suicidal behavior and vivid dreams. "The FDA is aware of the increasing number of reports, and we take them seriously," said spokesman Christopher DiFrancesco.
ISMP, is an organization that prepared the analysis has served hospitals and pharmacists for years as a clearinghouse for information on medication errors. Thomas J. Moore, a senior scientist with ISMP said, "We believe that one of the most important tools to promote is to monitor trends on a regular basis."
The FDA called for a stronger risk warning on the Chantix label.
Image Source: steadyhealth.com